What is 21 CFR Part 11?
The Code of Federal Regulations (CFR) are general and permanent rules published by the executive departments and agencies of the U.S. federal government. Title 21 of the CFR contains all regulations of the Food and Drug Administration (FDA) related to electronic signatures.
Part 11 of 21 CFR states the rules that FDA-regulated businesses must follow for their electronic documents and signatures to be considered equivalent to physical paper records and wet signatures.
Popular industries
- Drugs and pharmaceuticals
- Food and beverage
- Medical devices
- Tobacco products
- Cosmetics
- Radiation-emitting devices
- Animal and veterinary
- Vaccines, blood, and biologics
- Dietary supplements
- Food and color additives
If you don't comply with 21 CFR Part 11, you can face:
- Notices of inquiry
- Warning letters
- Initiation of disqualification proceedings
- Seizure of FDA-regulated products
- Criminal fines
- Recalls
- Retention orders
- Criminal prosecution
How to achieve FDA-compliant e-signatures with Zoho Sign
Zoho Sign helps FDA-regulated businesses use digital signatures instead of signing documents with pen and paper. Businesses can stay compliant with the following software features:
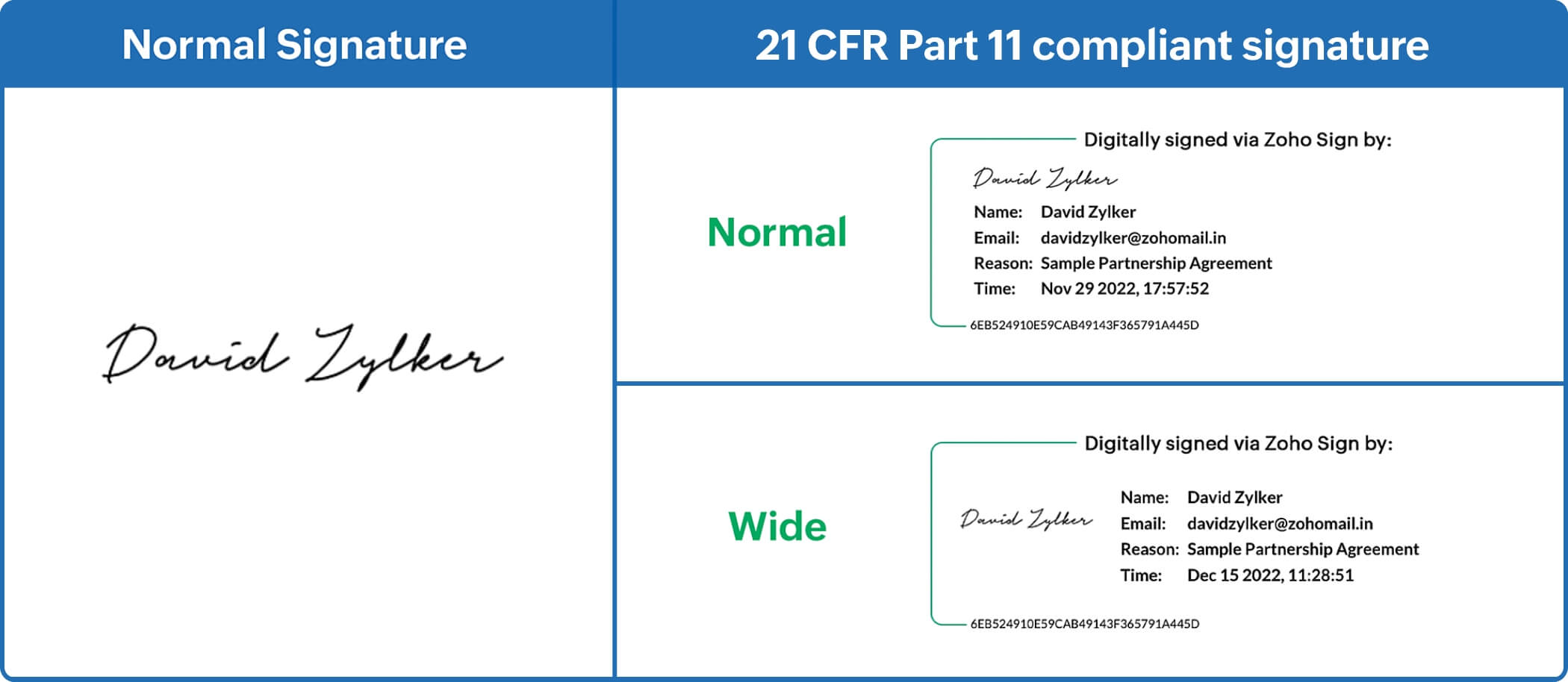
- Electronic signature with printed name of the signer
- Signing date and time, unique user ID, PKI cryptography, and signing reason
- Signer identity verification and authentication
- Customized legal disclosure
- Security, control, and validation
- Free onboarding and training sessions
Key benefits
- Single-click account setup and configuration
- Easy compliance with 21 CFR Part 11 module for electronic signatures
- Detailed audit trails and reports
How to enable Zoho Sign controls for 21 CFR Part 11
Pause
- STEP 1
Click Settings in the left navigation pane.
- STEP 2
Go to Account Settings.
- STEP 3
Toggle 21 CFR Part 11 to ON.


